Conquer tortuosity
Stent Coronario
SupraFlex Cruz
Stent Coronario de Struts de Cr-Co liberador de Fármaco Sirolimus sobre polímero reabsorbible multicapa.
Fuerza de empuje media en Newton para stents con longitud de 38 a 40 mm.
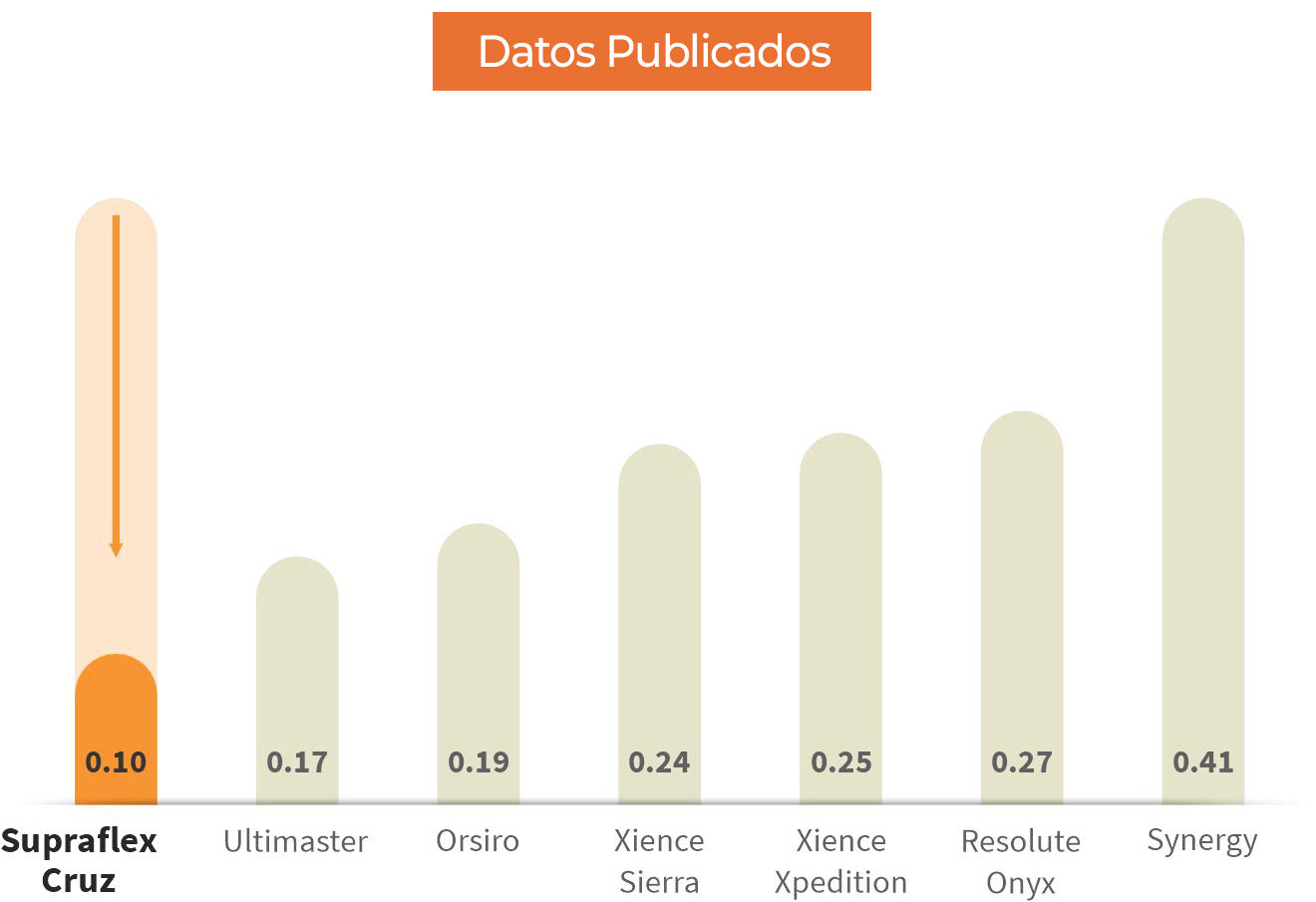
Los resultados de las pruebas comparativas pueden no ser necesariamente indicativos del rendimiento clínico. Prueba realizada y datos archivados en Sahajanand Medical Technologies Ltd. Pruebas realizadas en el sistema de stent Supraflex Cruz (2,50 x 40 mm) n=5, el sistema de stent Ultimaster (2,5 x 38 mm) n=4, el sistema de stent Orsiro (2,50 x 40 mm) n=5, sistema de stent Xience Sierra (2,5 x 38 mm) n=4, sistema de stent Xience Xpedition (2,5 x 38 mm) n=5, sistema de stent Resolute Onyx (2,5 x 38 mm) n=4, stent Synergy Sistem (2,5 x 38 mm) n=5. La prueba de rendimiento del catéter mide la fuerza promedio para cruzar un modelo de trayectoria compleja.
‘”Supraflex Cruz exhibió la mas baja fuerza de empuje media necesaria para pasar el dispositivo en comparación con otros DES ampliamente utilizados” - Datos publicados en Future Cardiology
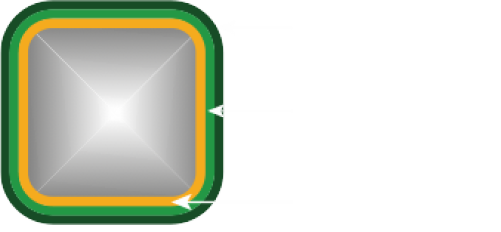
Enlace Patentado “LDZ”
- Mejora la flexibilidad de la Endoprótesis.
- Transmite la “Fuerza de Empuje” con mayor eficacia.
- Mejora la resistencia radial global.
- Resiste la compresión longitudinal.
Diseño de Celda Abierta
- Mejor Flexibilidad.
- Mejor acceso a las ramas laterales.
pioneros en las tecnologías de
polímeros biodegradables
Mezcla única de Polímeros Biodegradables hidrófilos-hidrófobos
- PLLA: Poly-L-Lactide (Hidrofóbico)
- PLCL: Poly L-Lactide-co-Caprolactone (Hidrofóbico)
- PVP: Polyvinyl Pyrrolidone (Hidrofílico)
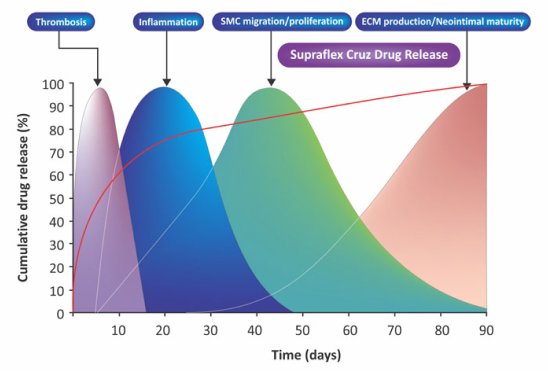
Aproximádamente el 80% del fármaco se libera en el primer mes. El fármaco restante está programado para liberarse durante los próximos 2 meses. Diseñado para cubrir todo el proceso de cicatrización de la pared vascular
Sección transversal del Strut
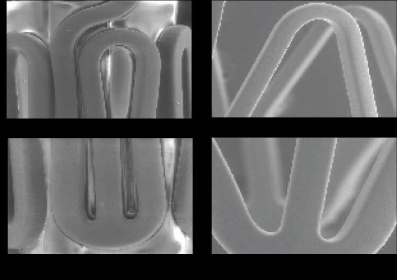
PATRÓN DE
CICATRIZACIÓN
OCT
NIH: Neointimal hyperplasia
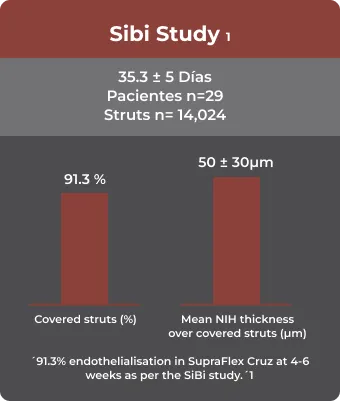
- Abhyankar, A, Abizaid, A, Chamié, D, Patel, G. Healing and early stent coverage after strut biodegradable polymer‐coated sirolimus‐eluting stent implantation: SiBi optical coherence tomography study. Catheter Cardiovasc Interv. 2020; 1– 8. https://doi.org/10.1002/ccd.29371
- Presented at EuroPCR 2019, 22 May 2019 12:15 – 13:15 Room 243 / Level 2
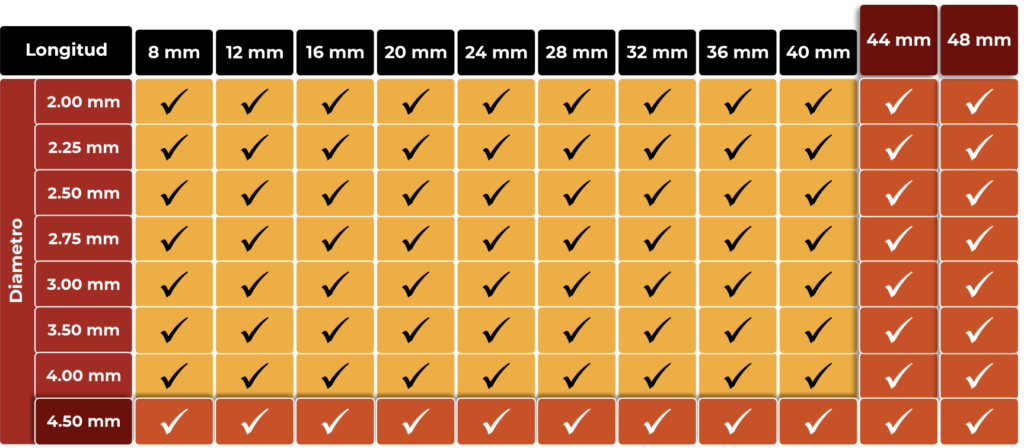
MUY AMPLIA GAMA DE TAMAÑOS PARA CUBRIR TODAS LAS NECESIDADES
LÍMITES DE SOBREEXPANSIÓN
| DIÁMETRO NOMINAL (MM) | LÍMITE DE POST-DILATACIÓN (MM) | |
|---|---|---|
| VASO PEQUEÑO (4 CORONAS) | 2.00/2.25 | 3.25 |
| VASO MEDIANO (6 CORONAS) | 2.50/2.75/3.00/3.50 | 4.25 |
| VASO GRANDE (8 CORONAS | 4.00/4.50 | 5.50 |
AMPLIA GAMA DE TAMAÑOS
| REFERENCIA | DESCRIPCIÓN | DIMENSIONES |
|---|---|---|
| FGTZ200008 | SUPRAFLEX CRUZ 2.00 MM X 08 MM | 2.00 MM X 08 MM |
| FGTZ200012 | SUPRAFLEX CRUZ 2.00 MM X 12 MM | 2.00 MM X 12 MM |
| FGTZ200016 | SUPRAFLEX CRUZ 2.00 MM X 16 MM | 2.00 MM X 16 MM |
| FGTZ200020 | SUPRAFLEX CRUZ 2.00 MM X 20 MM | 2.00 MM X 20 MM |
| FGTZ200024 | SUPRAFLEX CRUZ 2.00 MM X 24 MM | 2.00 MM X 24 MM |
| FGTZ200028 | SUPRAFLEX CRUZ 2.00 MM X 28 MM | 2.00 MM X 28 MM |
| FGTZ200032 | SUPRAFLEX CRUZ 2.00 MM X 32 MM | 2.00 MM X 32 MM |
| FGTZ200036 | SUPRAFLEX CRUZ 2.00 MM X 36 MM | 2.00 MM X 36 MM |
| FGTZ200040 | SUPRAFLEX CRUZ 2.00 MM X 40 MM | 2.00 MM X 40 MM |
| FGTZ200044 | SUPRAFLEX CRUZ 2.00 MM X 44 MM | 2.00 MM X 44 MM |
| FGTZ200048 | SUPRAFLEX CRUZ 2.00 MM X 48 MM | 2.00 MM X 48 MM |
| FGTZ225008 | SUPRAFLEX CRUZ 2.25 MM X 08 MM | 2.25 MM X 08 MM |
| FGTZ225012 | SUPRAFLEX CRUZ 2.25 MM X 12 MM | 2.25 MM X 12 MM |
| FGTZ225016 | SUPRAFLEX CRUZ 2.25 MM X 16 MM | 2.25 MM X 16 MM |
| FGTZ225020 | SUPRAFLEX CRUZ 2.25 MM X 20 MM | 2.25 MM X 20 MM |
| FGTZ225024 | SUPRAFLEX CRUZ 2.25 MM X 24 MM | 2.25 MM X 24 MM |
| FGTZ225028 | SUPRAFLEX CRUZ 2.25 MM X 28 MM | 2.25 MM X 28 MM |
| FGTZ225032 | SUPRAFLEX CRUZ 2.25 MM X 32 MM | 2.25 MM X 32 MM |
| FGTZ225036 | SUPRAFLEX CRUZ 2.25 MM X 36 MM | 2.25 MM X 36 MM |
| FGTZ225040 | SUPRAFLEX CRUZ 2.25 MM X 40 MM | 2.25 MM X 40 MM |
| FGTZ225044 | SUPRAFLEX CRUZ 2.25 MM X 44 MM | 2.25 MM X 44 MM |
| FGTZ225048 | SUPRAFLEX CRUZ 2.25 MM X 48 MM | 2.25 MM X 48 MM |
| FGTZ250008 | SUPRAFLEX CRUZ 2.25 MM X 08 MM | 2.50 MM X 08 MM |
| FGTZ250012 | SUPRAFLEX CRUZ 2.25 MM X 12 MM | 2.50 MM X 12 MM |
| FGTZ250016 | SUPRAFLEX CRUZ 2.25 MM X 16 MM | 2.50 MM X 16 MM |
| FGTZ250020 | SUPRAFLEX CRUZ 2.25 MM X 20 MM | 2.50 MM X 20 MM |
| FGTZ250024 | SUPRAFLEX CRUZ 2.25 MM X 24 MM | 2.50 MM X 24 MM |
| FGTZ250028 | SUPRAFLEX CRUZ 2.25 MM X 28 MM | 2.50 MM X 28 MM |
| FGTZ250032 | SUPRAFLEX CRUZ 2.25 MM X 32 MM | 2.50 MM X 32 MM |
| FGTZ250036 | SUPRAFLEX CRUZ 2.25 MM X 36 MM | 2.50 MM X 36 MM |
| FGTZ250040 | SUPRAFLEX CRUZ 2.25 MM X 40 MM | 2.50 MM X 40 MM |
| FGTZ250044 | SUPRAFLEX CRUZ 2.25 MM X 44 MM | 2.50 MM X 44 MM |
| FGTZ250048 | SUPRAFLEX CRUZ 2.25 MM X 48 MM | 2.50 MM X 48 MM |
| FGTZ275008 | SUPRAFLEX CRUZ 2.75 MM X 08 MM | 2.75 MM X 08 MM |
| FGTZ275012 | SUPRAFLEX CRUZ 2.75 MM X 12 MM | 2.75 MM X 12 MM |
| FGTZ275016 | SUPRAFLEX CRUZ 2.75 MM X 16 MM | 2.75 MM X 16 MM |
| FGTZ275020 | SUPRAFLEX CRUZ 2.75 MM X 20 MM | 2.75 MM X 20 MM |
| FGTZ275024 | SUPRAFLEX CRUZ 2.75 MM X 24 MM | 2.75 MM X 24 MM |
| FGTZ275028 | SUPRAFLEX CRUZ 2.75 MM X 28 MM | 2.75 MM X 28 MM |
| FGTZ275032 | SUPRAFLEX CRUZ 2.75 MM X 32 MM | 2.75 MM X 32 MM |
| FGTZ275036 | SUPRAFLEX CRUZ 2.75 MM X 36 MM | 2.75 MM X 36 MM |
| FGTZ275040 | SUPRAFLEX CRUZ 2.75 MM X 40 MM | 2.75 MM X 40 MM |
| FGTZ275044 | SUPRAFLEX CRUZ 2.75 MM X 44 MM | 2.75 MM X 44 MM |
| FGTZ275048 | SUPRAFLEX CRUZ 2.75 MM X 48 MM | 2.75 MM X 48 MM |
| FGTZ300008 | SUPRAFLEX CRUZ 3.00 MM X 08 MM | 3.00 MM X 08 MM |
| FGTZ300012 | SUPRAFLEX CRUZ 3.00 MM X 12 MM | 3.00 MM X 12 MM |
| FGTZ300016 | SUPRAFLEX CRUZ 3.00 MM X 16 MM | 3.00 MM X 16 MM |
| FGTZ300020 | SUPRAFLEX CRUZ 3.00 MM X 20 MM | 3.00 MM X 20 MM |
| FGTZ300024 | SUPRAFLEX CRUZ 3.00 MM X 24 MM | 3.00 MM X 24 MM |
| FGTZ300028 | SUPRAFLEX CRUZ 3.00 MM X 28 MM | 3.00 MM X 28 MM |
| FGTZ300032 | SUPRAFLEX CRUZ 3.00 MM X 32 MM | 3.00 MM X 32 MM |
| FGTZ300036 | SUPRAFLEX CRUZ 3.00 MM X 36 MM | 3.00 MM X 36 MM |
| FGTZ300040 | SUPRAFLEX CRUZ 3.00 MM X 40 MM | 3.00 MM X 40 MM |
| FGTZ300044 | SUPRAFLEX CRUZ 3.00 MM X 44 MM | 3.00 MM X 44 MM |
| FGTZ300048 | SUPRAFLEX CRUZ 3.00 MM X 48 MM | 3.00 MM X 48 MM |
| FGTZ350008 | SUPRAFLEX CRUZ 3.50 MM X 08 MM | 3.50 MM X 08 MM |
| FGTZ350012 | SUPRAFLEX CRUZ 3.50 MM X 12 MM | 3.50 MM X 12 MM |
| FGTZ350016 | SUPRAFLEX CRUZ 3.50 MM X 16 MM | 3.50 MM X 16 MM |
| FGTZ350020 | SUPRAFLEX CRUZ 3.50 MM X 20 MM | 3.50 MM X 20 MM |
| FGTZ350024 | SUPRAFLEX CRUZ 3.50 MM X 24 MM | 3.50 MM X 24 MM |
| FGTZ350028 | SUPRAFLEX CRUZ 3.50 MM X 28 MM | 3.50 MM X 28 MM |
| FGTZ350032 | SUPRAFLEX CRUZ 3.50 MM X 32 MM | 3.50 MM X 32 MM |
| FGTZ350036 | SUPRAFLEX CRUZ 3.50 MM X 36 MM | 3.50 MM X 36 MM |
| FGTZ350040 | SUPRAFLEX CRUZ 3.50 MM X 40 MM | 3.50 MM X 40 MM |
| FGTZ350044 | SUPRAFLEX CRUZ 3.50 MM X 44 MM | 3.50 MM X 44 MM |
| FGTZ350048 | SUPRAFLEX CRUZ 3.50 MM X 48 MM | 3.50 MM X 48 MM |
| FGTZ400008 | SUPRAFLEX CRUZ 4.00 MM X 08 MM | 4.00 MM X 08 MM |
| FGTZ400012 | SUPRAFLEX CRUZ 4.00 MM X 12 MM | 4.00 MM X 12 MM |
| FGTZ400016 | SUPRAFLEX CRUZ 4.00 MM X 16 MM | 4.00 MM X 16 MM |
| FGTZ400020 | SUPRAFLEX CRUZ 4.00 MM X 20 MM | 4.00 MM X 20 MM |
| FGTZ400024 | SUPRAFLEX CRUZ 4.00 MM X 24 MM | 4.00 MM X 24 MM |
| FGTZ400028 | SUPRAFLEX CRUZ 4.00 MM X 28 MM | 4.00 MM X 28 MM |
| FGTZ400032 | SUPRAFLEX CRUZ 4.00 MM X 32 MM | 4.00 MM X 32 MM |
| FGTZ400036 | SUPRAFLEX CRUZ 4.00 MM X 36 MM | 4.00 MM X 36 MM |
| FGTZ400040 | SUPRAFLEX CRUZ 4.00 MM X 40 MM | 4.00 MM X 40 MM |
| FGTZ400044 | SUPRAFLEX CRUZ 4.00 MM X 44 MM | 4.00 MM X 44 MM |
| FGTZ400048 | SUPRAFLEX CRUZ 4.00 MM X 48 MM | 4.00 MM X 48 MM |
| FGTZ450008 | SUPRAFLEX CRUZ 4.50 MM X 08 MM | 4.50 MM X 08 MM |
| FGTZ450012 | SUPRAFLEX CRUZ 4.50 MM X 12 MM | 4.50 MM X 12 MM |
| FGTZ450016 | SUPRAFLEX CRUZ 4.50 MM X 16 MM | 4.50 MM X 16 MM |
| FGTZ450020 | SUPRAFLEX CRUZ 4.50 MM X 20 MM | 4.50 MM X 20 MM |
| FGTZ450024 | SUPRAFLEX CRUZ 4.50 MM X 24 MM | 4.50 MM X 24 MM |
| FGTZ450028 | SUPRAFLEX CRUZ 4.50 MM X 28 MM | 4.50 MM X 28 MM |
| FGTZ450032 | SUPRAFLEX CRUZ 4.50 MM X 32 MM | 4.50 MM X 32 MM |
| FGTZ450036 | SUPRAFLEX CRUZ 4.50 MM X 36 MM | 4.50 MM X 36 MM |
| FGTZ450040 | SUPRAFLEX CRUZ 4.50 MM X 40 MM | 4.50 MM X 40 MM |
| FGTZ450044 | SUPRAFLEX CRUZ 4.50 MM X 44 MM | 4.50 MM X 44 MM |
| FGTZ450048 | SUPRAFLEX CRUZ 4.50 MM X 48 MM | 4.50 MM X 48 MM |
ESTUDIOS CLÍNICOS
– Healing and early stent coverage after ultrathin strut biodegradable polymer-coated sirolimus-eluting stent implantation: SiBi optical coherence tomography study Abhyankar A, Abizaid A, Chamie D, et al. Catheter Cardiovasc lnterv. 2020 Nov 28. doi: 10.1002/ccd.29371.
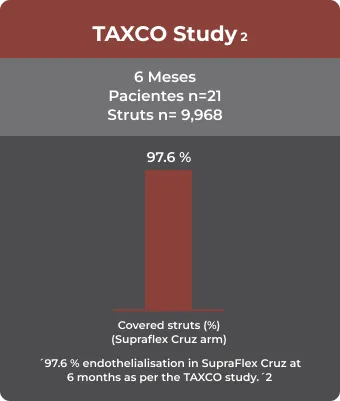
– Comparison of neointimal coverage between ultrathin biodegradable polymer-coated sirolimus-eluting stents and durable polymer-coated everolimus-eluting stents: 6 months optical coherence tomography follow-up from the TAXCO study Abhyankar A, Abizaid A, Chamie D, et al. Catheter Cardiovasc lnterv. 2021 Feb 15;97(3):423-30.
– Clinical outcomes in 995 unselected real-world patients treated with an ultrathin biodegradable polymer-coated sirolimus-eluting stent: 12-month results from the FLEX. Registry Lemos PA, Chandwani P, Saxena S, et al. BMJ Open. 2016 Feb 17;6{2):e010028. doi: 10.1136/bmjopen- 2015-010028.
– Early vascular healing with biodegradable polymer coated sirolimus-eluting coronary stent implantation: assessed by optical coherence tomography results at 4-month follow-up Abhyankar A, Prajapati J, Reddy S, et al. Minerva Cardioangiol. 2013 Jun;61(3):313-22.
– A prospective multicenter randomized all-comers trial to assess the safety and effectiveness of the thin-strut sirolimuseluting coronary stent SUPRAFLEX: rationale and design of the TALENT trial Modolo R, Chichareon P, Kogame N, et al. Eurolntervention. 2019 Jul 20;15(4):e362-e369.
– Safety and efficacy of a sirolimus-eluting coronary stent with ultra-thin strut for treatment of atherosclerotic lesions (TALENT): a prospective multicentre randomised controlled trial Zaman A, de Winter RJ, Kogame N, et al. Lancet. 2019 Mar 9;393(10175):987-97.
– Prospective Multicenter Randomized All-Comers Trial to Assess the Safety and Effectiveness of the Ultra-Thin Strut Sirolimus-Eluting Coronary Stent Supraflex: Two-Year Outcomes of the TALENT Trial Gao C, Kogame N, Sharif F, et al. Circ Cardiovasc lnterv. 2021 Mar 9: CIRCINTERVENTIONS120010312. doi: 10.1161/CIRCINTERVENTIONS.120.010312
– A randomized controlled trial to compare the safety and efficacy of sirolimus-eluting biodegradable polymer ultra-thin stent (SUPRAFLEX Cruz) and everolimus-eluting biodegradable polymer stent (SYNERGY) in treatment for three-vessel coronary artery disease: design of the Multivessel TALENT trial Hara H, Gao C, Kogame N, et al. Eurolntervention. 2020 Dec 18;16(12):e997-e1004.
– Physiology-guided revascularization versus optimal medical therapy of nonculprit lesions in elderly patients with myocardial infarction: Rationale and design of the FIRE trial Biscaglia S, Guiducci V, Santarelli A, et al. Am Heart J. 2020 Nov;229:100-109.
– Very late outcomes of drug-eluting stents coated with biodegradable polymers: insights from the 5-year follow-up of the randomized PAINT trial Marchini JF, Gomes WF, Moulin B, et al. Cardiovasc Diagn Ther. 2014 Dec;4(6):480-6.
– Late clinical outcomes after implantation of drug-eluting stents coated with biodegradable polymers: 3-year follow-up of the PAINT randomized trial Lemos PA, Moulin B, Perin MA, et al. Eurolntervention. 2012 May 15;8(1):117-9.
– Randomized evaluation of two drug-eluting stents with identical metallic platform and biodegradable polymer but different agents (Paclitaxel or Sirolimus) compared against bare stents: 1-Year results of the PAINT trial Lemos PA, Moulin B, Perin MA, et al. Catheter Cardiovasc lnterv. 2009 Nov 1;74(5):665-73.
– Prospective evaluation of an ultrathin strut biodegradable polymer-coated sirolimus-eluting stent: 12 months’ results from the S-FLEX UK registry Choudhury A, Garg S, Smith J, et al. BMJ Open. 2019 Oct 11;9(10):e026578. doi: 10.1136/ bmjopen-2018-026578.
– Clinical outcomes in 995 unselected real-world patients treated with an ultrathin biodegradable polymer-coated sirolimus-eluting stent: 12-month results from the FLEX Registry Lemos PA, Chandwani P, Saxena S, et al. BMJ Open. 2016 Feb 17;6(2):e010028. doi: 10.1136/bmjopen-2015-010028.
– The ultra-thin strut sirolimus-eluting coronary stent: SUPRAFLEX doi 10.2217/fca-2019-0083. Device evaluation. Future of Cardiology
– A randomised controlled trial of the sirolimus-eluting biodegradable polymer ultra-thin Supraflex stent versus the everolimus-eluting biodegradable polymer SYNERGY stent for three-vessel coronary artery disease: rationale and design of the Multivessel TALENT trial. Eurointervention 2020 Dec 18;16(12):e997-e1004. DOI: 10.4244/EIJ-D-20-00772.
– Twelve months clinical outcomes of “Nano-crush technique” for the treatment of bifurcation lesions using ultra-thin (60 μm) sirolimus-eluting coronary stents. Minerva Cardiol Angiol. 2022 Feb 25. doi: 10.23736/S2724-5683.21.05875-0.
– Real-World Use Of Ultrathin-Strut Biodegradable Polymer-Coated Sirolimus-Eluting Stents In Patients With Coronary Artery Disease: 6-Month Clinical Outcomes Vasc Health Risk Manag 2019 Oct 18;15:439-447.Prakash Ajmera 1, Ramesh Pothineni 2, Kamal Kumar Chawla 1, Sai Sudhakar Mantravadi 3, Pankaj Vinod Jariwala 4, Vinod Vijan 5, Vikrant Vijan 5 DOI: 10.2147/VHRM.S200699.
– Clinical outcomes and complications of treatment with supraflex stent in patients with coronary artery disease: One-year follow-up. Eur J Transl Myol. 2019 PMID: 313549 PMCID: PMC6615074DOI: 10.4081/ejtm.2019.8231.
– Comparison of Clinical Outcomes Following Single versus Multivessel Percutaneous Coronary Intervention Using Biodegradable Polymer Coated Sirolimus-Eluting Stent in an All-comers Patient Population. Cardiovasc Hematol Agents Med Chem. 2016;14(1):39-48.
– “Twelve months clinical outcomes of Nano Crush Technique for the treatment of bifurcation lesions using ultra-thin (60 micras) Sirolimus eluting coronary stents. Minerva cardiology and angiology 2022 feb 25, DOI: 10.23736/S2724-5683.21.05875-0”.
– Side-branch expansion capacity of contemporary DES platforms. Öner et al. Eur J Med Res https://doi.org/10.1186/s40001-021-00595-7.
– Long term outcomes of ultrathin versus standard thickness second-generation drug eluting stents: Meta-analysis of randomized trials. Catheter Cardiovasc Interv. 2021;1–12. DOI: 10.1002/ccd.29866
– Long-termfollow-up after ultrathin vs conventional 2nd-generation drug-eluting stents: a systematic review and meta-analysis of randomized controlled trials. European Heart Journal (2021) 00, 1–12. doi:10.1093/eurheartj/ehab280.
– Clinical Outcomes of Biodegradable Polymer-coated Ultrathin Strut Sirolimus-eluting Stents in a Real-world Patient Population: T-Flex Registry Three-year Results with High-Risk Subgroups.
Abstracts of EuroPCR 2022, Scientific Abstract e-Book (Euro22A-POS171). Type: Registry View Link
– Prospective Evaluation of Ultrathin-strut Biodegradable Polymer-coated Sirolimus-eluting Stents in an All-comers Patient Population: Slovakia Registry with a Subgroup Analysis.
Abstracts of EuroPCR 2022, Scientific Abstract e-Book (Euro22A-POS147). Type: Registry View Link
– Evaluation of Novel Ultrathin, Biodegradable Polymer-Coated Tetriflex Sirolimus-Eluting Stent Optimization Using Intravascular Ultrasound (IVUS) in Short Coronary Lesions (<20 mm) vs. Long Coronary Lesions (≥20 mm): Tetriflex IVUS Study.
Abstracts of EuroPCR 2022, Scientific Abstract e-Book (Euro22A-POS228).
Type: Intravascular Ultrasound Study View Link
– Twelve months clinical outcomes of “Nano-crush technique” for the treatment of bifurcation lesions using ultra-thin (60 μm) sirolimus-eluting coronary stents.
Minerva Cardiol Angiol. 2022 Feb 25. doi: 10.23736/S2724-5683.21.05875-0.
Type: Registry View Link
– Clinical outcomes of ultrathin biodegradable polymer-coated sirolimus-eluting stents in an all-comer population: One-year results from the T-FLEX registry including high-risk subgroups.
Anatol J Cardiol . 2021 Oct;25(10):706-715. Type: Registry View Link
– Prospective evaluation of the Supraflex Family sirolimus-eluting coronary stent system in a ‘real-world’ patient population: Interim analysis from the S-FLEX Netherlands Registry.
Abstracts of EuroPCR 2021, Scientific Abstract e-Book (Euro21A-POS251). Type: Registry View Link
– Ultrathin Sirolimus-eluting Stent in Real-world CAD patients: 12 months.
Results Abstracts of EuroPCR 2021, Scientific Abstract e-Book (Euro21A-POS276). Type: Registry View Link
– Ultrathin (60 μm), ultralong (≥40 mm) sirolimus-eluting stent: study of clinical and safety profiles among real-world patients.
Anatol J Cardiol. 2021 Feb;25(2):111-119. Type: Registry View Link
– Healing and early stent coverage after ultrathin strut biodegradable polymer-coated sirolimus-eluting stent implantation: SiBi optical coherence tomography study.
Catheter Cardiovasc Interv. 2021 Dec 1;98(7):1335-1342. Type: Optical Coherence Tomography Study View Link
– Comparison of neointimal coverage between ultrathin biodegradable polymer-coated sirolimus-eluting stents and durable polymer-coated everolimus-eluting stents: 6 months optical coherence tomography follow-up from the TAXCO study.
Catheter Cardiovasc Interv. 2021 Feb 15;97(3):423-430.
Type: Optical Coherence Tomography Study View Link
– Twelve months clinical outcomes after implantation of long ultra-thin strut biodegradable polymer-coated sirolimus-eluting stents in atherosclerotic coronary lesions.
Abstracts of PCR e-Course 2020, Vol. 16, Suppl. AC, June 2020 (Euro20A-POS703) Type: Registry View Link
– Performance of ultra-thin strut biodegradable polymer-coated sirolimus-eluting stent in females with coronary artery disease: results of twelve months follow-up.
Abstracts of PCR e-Course 2020, Vol. 16, Suppl. AC, June 2020 (Euro20A-POS702). Type: Registry View Link
– Clinical performance of ultra-thin strut biodegradable polymer-coated sirolimus-eluting stents in young patients with coronary artery disease: results of twelve months outcomes.
Abstracts of PCR e-Course 2020, Vol. 16, Suppl. AC, June 2020 (Euro20A-POS699). Type: Registry View Link
– Evaluation of ultra-thin strut biodegradable polymer-coated sirolimus-eluting stent in patients with small coronary arteries (≤2.5 mm).
Abstracts of PCR e-Course 2020, Vol. 16, Suppl. AC, June 2020 (Euro20A-POS651). Type: Registry View Link
– Evaluation of clinical outcomes after ultra-thin strut (60 µm) biodegradable polymer-coated sirolimus-eluting stent implantation in patients with acute coronary syndrome.
Abstracts of PCR e-Course 2020, Vol. 16, Suppl. AC, June 2020 (Euro20A-POS650). Type: Registry View Link
– One-year clinical outcomes of ultrathin biodegradable polymer coated sirolimus eluting stents in total occlusion: a multicentre, all-comer analysis.
Abstracts of PCR e-Course 2020, Vol. 16, Suppl. AC, June 2020 (Euro20A-OP124) Type: Registry View Link
– One-year clinical outcomes of ultrathin biodegradable polymer coated sirolimus eluting stents for multivessel treatment.
Abstracts of PCR e-Course 2020, Vol. 16, Suppl. AC, June 2020 (Euro20A-POS646). Type: Registry View Link
– One-year clinical outcomes of ultra-thin strut (60 μm) biodegradable polymer-coated sirolimus-eluting coronary stents in diabetic patients.
Abstracts of PCR e-Course 2020, Vol. 16, Suppl. AC, June 2020 (Euro20A-POS624). Type: Registry View Link
– Twelve-month clinical outcomes of an ultra-thin (60 μm) strut, biodegradable polymer-coated, sirolimus-eluting coronary stent in STEMI patients – A real-world, multi-centre experience.
Abstracts of PCR e-Course 2020, Vol. 16, Suppl. AC, June 2020 (Euro20A-POS623). Type: Registry View Link
– Clinical outcomes of biodegradable polymer-coated ultrathin strut sirolimuseluting stents in a “real-world” patient population: two-year results from the T-flex registry.
Abstracts of PCR e-Course 2020, Vol. 16, Suppl. AC, June 2020 (Euro20A-POS652) Type: Registry View Link
– Physiology-guided revascularization versus optimal medical therapy of nonculprit lesions in elderly patients with myocardial infarction: Rationale and design of the FIRE trial.
Am Heart J. 2020 Nov;229:100-109. Type: Randomized Controlled Trial View Link
– A randomised controlled trial of the sirolimus-eluting biodegradable polymer ultra-thin Supraflex stent versus the everolimus-eluting biodegradable polymer SYNERGY stent for three-vessel coronary artery disease: rationale and design of the Multivessel TAL.
EuroIntervention. 2020 Dec 18;16(12):e997-e1004. Type: Randomized Controlled Trial View Link
– Real-world use of ultrathin-strut biodegradable polymer-coated sirolimus-eluting stents in patients with coronary artery disease: 6-month clinical outcomes.
Vasc Health Risk Manag. 2019;15:439–447 Type: Registry View Link